How does Ash2™Salt work?
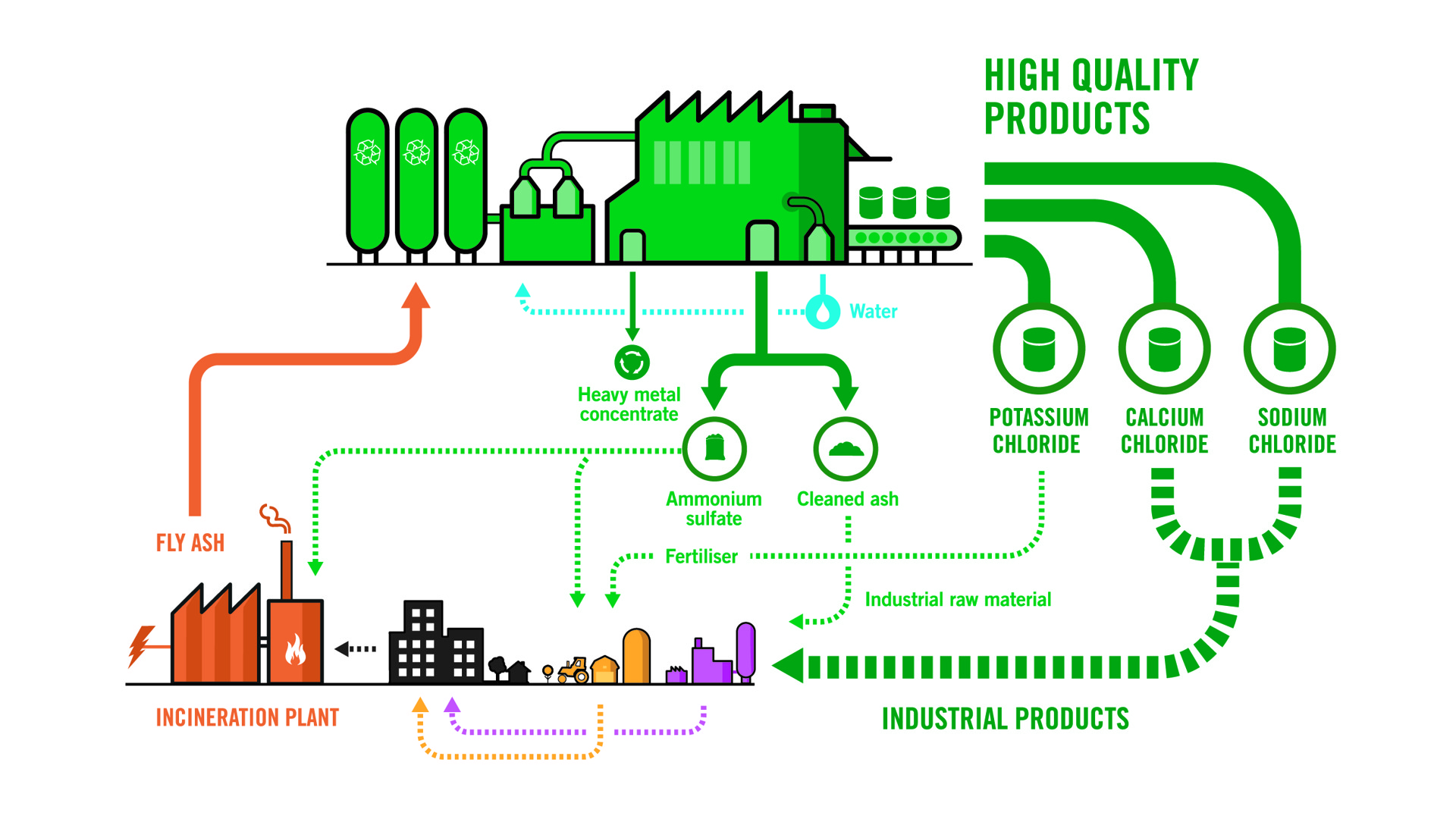
When flue gases are purified during waste incineration, fly ash is generated - a hazardous waste with high levels of heavy metals and chlorides. In the Ash2Salt process, the fly ash is washed, and three commercial salts are extracted from the wash liquid: sodium chloride, potassium chloride, and calcium chloride.
The process is preceded by two pre-treatment steps. In the first step, fly ash is washed with water resulting in a cleaned ash and a leachate containing mainly chloride salts and heavy metals that continues to the second step.
In the second step, sulphides are used to precipitate heavy metals from the leachate and the resulting chloride-saturated liquid then continues to the Ash2Salt process.
The Ash2Salt process separates calcium chloride (CaCl2), potassium chloride (KCl) and sodium chloride (NaCl) by the use of a single evaporator. In addition, water extracted from the evaporator is recirculated to the initial washing step.
In a washing plant for fly ash without the Ash2Salt process, following limitations exist:
- The washing plant must have permission to discharge large amounts of chloride-containing effluents
- The chloride content of the ash can vary depending on what the incineration plants burn resulting in different amounts of chloride effluent that must be discharged.
- Fresh water is needed for the washing
The main advantages of having the Ash2Salt process are:
- Washing of fly ash without a discharge of chlorides
- High tolerance for variations in chloride content in the ash
- Creates a source of income by producing pure commercial salts and ammonia
- Recovers nitrogen and potassium, which can be used in fertiliser production
- No need for fresh water since the Ash2Salt process can use landfill leachate and process water for the washing